REVERSE OSMOSIS
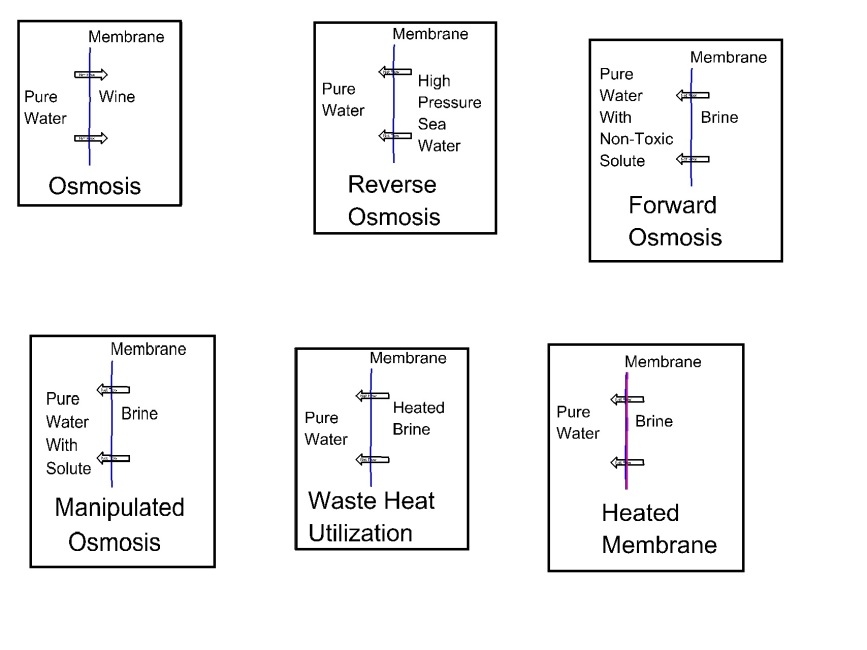
The Osmosis experiment has intrigued many people for years. Water flowed uphill through a membrane into wine. Water flow stops when a high pressure (osmotic pressure) is applied to the wine. Further increases in pressure on the wine will cause pure water to flow from the wine to the water. This is known as reverse osmosis.
We now know that water vapor pressure is the driving force for Osmotic flow and that the small pore size in the membrane blocks larger solutes in the wine (sea water), “we enjoy the wine without modification”. From Raoult’s Law, we know that the solutes in the wine lowered the water vapor pressure in the wine. Thus flow from the water into the wine. From thermodynamics, increased pressure on the wine caused the water vapor pressure to increase. Thus, net water flow between water and the wine stopped. Further increase in the applied pressure on the wine, increases the water vapor pressure and causes water from the sea water (wine) to flow through the membrane into the pure water side. We note that water vapor pressure also increases with rising temperature.
After years of work, a synthetic membrane was developed in 1959 by Sidney Loeb and Srinivasa Sourirajan at UCLA. Reverse Osmosis was then promoted as a feasible means to produce fresh water from sea water (brine) under the President Kennedy administration.
Commercial RO (reverse osmosis) systems operate up to 1000 psi (6900 kPa) psi applied pressure on the brine side to force pure water extraction. The specific design of each RO system depends on the feedwater impurities and the freshwater requirements. According to manufacturer’s literature home RO systems use conventional water pressure of approximately 50 psi (345 kPa) with “filters” to remove up to 99% of contaminants in the tap water.
More recently several innovations for reverse osmosis methods have been introduced. They manipulate the vapor pressure of the brine solution or the pure water side of the membrane to extract pure water from the brine.
A Forward Osmosis system lowers the vapor pressure of the pure water side by introducing a non-toxic, recoverable solute into the pure water. This lowers the water vapor pressure thus draws pure water from the brine through the membrane.
Manipulated Osmosis lowers the vapor pressure of the pure water with a non-potable solute. The resulting purified water is used for industrial processes.
Others have used waste heat to increase the temperature (and water vapor pressure) of the brine solution this increases water flow across the membrane. The resulting purified water must be carefully monitored since increased brine temperature also tends to increase the membrane temperature, and thus increase the pore size in the membrane. Others are developing a heated membrane. The energy requirement is reduced since only a small portion of the brine is heated. Additionally, others are working on various membrane improvements.