OSMOTIC PRESSURE
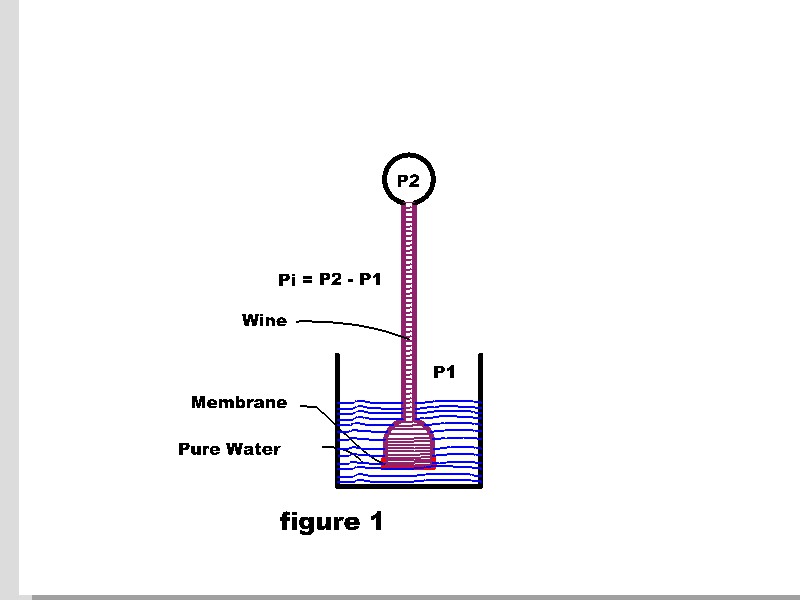
JA Nollet’s simple experiment (1748) to investigate the filter efficiency of a bladder membrane produced an enigma which is still misunderstood by many persons today. Wine in a column was separated from pure water by a pig’s bladder membrane (figure 1). The static pressure produced by a column of wine was expected to force the wine through the membrane into the pure water. Traces of color in the pure water would give an indication of the filter efficiency of the membrane.
Instead, pure water flowed upward through the membrane into the wine. And, a significant pressure applied to the wine was required to halt the flow. Many others repeated the experiment, including Pfeffer, de Vries, Donders, Hamburger, Soret, Raoult, and others. They found that replacing wine with a sugar/water solution produced the same results. In fact, the results were repeated with solutes other than sugar. The pressure required to stop the flow of pure water into the solution “osmotic pressure” increased with solute concentration.
J. van’t Hoff (1888), compared the experimental results with the newly discovered thermodynamic behavior of low pressure gases. After one hundred fifty years, this was the first thermodynamic justification of osmotic pressure. He did not explain the osmosis process, and there was some problem that flow through the membrane was from a low osmotic pressure region to a higher osmotic pressure region.
He was awarded the first Nobel prize in chemistry in 1901 “for his discovery of the laws of chemical dynamics and osmotic pressure in solutions”. His work has been challenged by many. We note the significance of van’t Hoff’s work. After one hundred fifty years, the measured phenomenon of osmotic pressure was supported with thermodynamic theory. We note that osmotic pressure is only required to stop the flow.
The magnitude of osmotic pressure is usually not important, rather, we are interested only in the direction of flow and the effects of osmosis. Osmosis has many applications including dehydration: Dried produce can be safely stored for longer periods. Brine or sugar solutions are used to dehydrate meats which then can be safely stored for 2 -3 months without refrigeration. Fruits can be partially dried a sugar syrup coating.
Osmosis processes have proved useful for water purification. Pure water naturally flows through a membrane into polluted water. Reverse osmosis processes increase pressure on the polluted water. With the proper membrane, pure water only flows through the membrane from the high-pressure side. This has found application for purifying sea water. An under-sink application produces a small amount of purified water from tap water. Other applications of osmosis are also used.
Osmosis is important in biological and medical applications. Cells are surrounded by body fluids. The difference in solute concentration inside and outside of the cell is vitally important. In a neutral (isotonic) environment, the cell does not exchange water with body fluids. For hypertonic fluids, water flows out through the cell membrane to cause cell dehydration. Hypotonic solutions cause water to enter cells and may burst a cell. This knowledge is important for storage of blood and body parts and for injected medicines.
“The Molecular Theory of Osmosis by LD Howlett” explains osmosis and osmotic pressure in more detail and introduces a molecular model for osmotic flow through a membrane.
2 Replies to “Osmotic Pressure”
I like what you guys tend to be up too. This kind of clever work and exposure!
Keep up the wonderful works guys I’ve incorporated you guys to my
own blogroll.
Keep up the superb work, I read few posts on this website and I believe that your blog is very
interesting and has bands of good information.