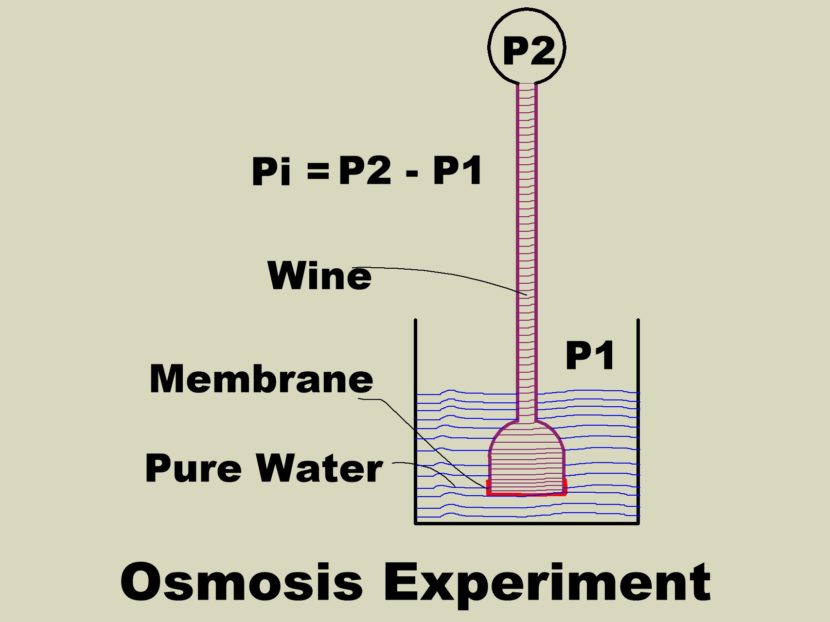
Osmosis is the movement of a solvent across a semi permeable membrane into a more dilute solution of the solvent. The semi permeable membrane prevents the solute from passing through the membrane. The original osmosis experiment used water and wine. Later experimenters found the same behavior using a sugar water solution instead of wine. Various membranes were used that allowed the passage of water molecules but did not allow the larger sugar molecules to pass.
We proposed that free water molecules exist in a liquid. The concentration of these free molecules is related to the vapor pressure. (Ref. 1) Note that this concept differs from others that suggest vapor pressure exists beyond the liquid surface. The effective vapor pressure of water is lowered buy the addition of a solute (Raoult’s law). Therefore, free water molecules move from the pure water, through the membrane, to the sugar water solution. (from a higher vapor pressure to a lower vapor pressure region.)
Diffusion is a movement of particles from a higher concentration to a lower concentration region. (Fick’s law)
For a gas, we know that pressure is a measure of the total impact between the gas molecules and a surface. Atmospheric pressure is the sum of the partial pressure of each gas component in the atmosphere. (patm = poxygen + pnitrogen + ….) Now if we introduce, in a corner, a high concentration of oxygen we know that it will quickly diffuse until the concentration of oxygen becomes uniform in the entire region. Oxygen molecules move from a high concentration region to a lower concentration region. Since partial pressure is directly related to the concentration of each gas, we can also say that diffusion occurs from a high partial pressure region to a lower partial pressure region.
Therefore, the molecular movements of osmosis and diffusion are the same. Free molecules move from a higher concentration region to a lower concentration region. (from higher pressure region to a lower pressure region.) In engineering, we commonly think of force or pressure as the driving mechanism. In chemistry, the number of molecules (concentration) is important for reactions.
We therefore conclude that osmosis and diffusion are the same.. Both occur due to molecular motion.. Molecules move from a high concentration (pressure) region to a lower concentration (pressure) region.. For osmosis, a membrane separates the two regions.
- Osmosis: The Molecular Theory, Larry D. Howlett, 2013
© Larry Howlett HTMD Engineering 2020
HTMD Engineering Provides Engineering Solutions for Todays Challenges. We deliver new designs and / or improve existing processes and equipment.
We would like to bid on your projects. 815-766-3471 LDH@HTMDengineering.com